EGFR / ERBB1 / HER1 - SPR Binding Assay
Our service for comprehensive kinetic characterization of your small molecule kinase inhibitors
Theoretical background: The epidermal growth factor receptor (EGFR) is part of the erbB family of receptor tyrosine kinases, including HER2/erbB2, HER3/erbB3 and HER4/erbB4. Activation of ErbB receptors is initiated by ligand binding to their extracellular domain, leading to the formation of both, homo- and heterodimeric ErbB receptor complexes. Upon dimerization the receptor kinase undergoes autophosphorylation at specific tyrosine residues within the intracellular domain, acting as binding sites for proteins e.g. Shc, Grb2 resulting in activation of signaling pathways such as Ras-ERK, phosphoinositide-3 kinase, Src, and STAT. Deregulated EGFR signaling, caused by overexpression of the receptor or an enhanced ligand production, is frequent in many tumor types.
EGFR and inhibitors: Different drug classes targeting EGFR have been developed. Monoclonal antibodies are able to bind the extracellular ligand-binding domain (Cetuximab, Panitumumab) inducing receptor internalization and degradation. Small molecules targeting the tyrosine kinase domain (Gefitinib and Erlotinib, IC50 = 23 nM [1] and 2 nM [2]) prevent signal transduction and have shown promise as chemotherapeutic agents.
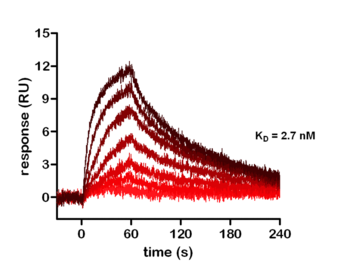
Figure: Real-time kinetic analysis of kinase inhibitor Erlotinib binding to EGFR using surface plasmon resonance.
[1] Barker AJ et al. (2001) Bioorg Med Chem Lett. 23;11(14):1911-4.
[2] Moyer JD et al. (1997) Cancer Res. 57(21):4838-48.
Please contact our application specialists to obtain more information and an individual quote tailored to your specific needs.
Tel.: +49 (0) 561-804 4661 | Fax: +49 (0) 561-804 4665 | info@biaffin.de
We value your privacy
In order to optimize our website for you and to be able to continuously improve it, we use cookies. Further information is available in our Data Protection Statement. Here you can find our Legal Information.