ADME
Do you want to specify the pharmacological potency of your compounds ?
Biaffin offers a rapid assay for ranking drugs according their ability to interact with the most abundant plasma proteins.
The entireties of physiological parameters that have to be considered while assessing the effectiveness of a drug are known as ADME (Adsorption, Distribution, Metabolism and Elimination). BIA technology provides a powerful tool for a cost-effective in vitro characterisation of potential drug candidates in early ADME studies.
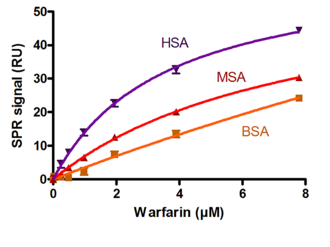
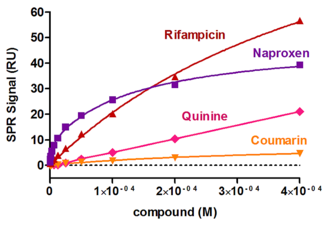
Biaffin offers a well-established rapid throughput assay for ranking drugs according their ability to interact with the most abundant plasma proteins (such as serum albumin, alpha 1-acid glycoprotein or gamma globulins), limiting their availability and pharmacological potency. To evaluate the binding strength of the clients drug compound a set of marker drugs with well-documented binding properties were analyzed too. A more detailed compound characterization of the high binding drugs including calculation of the affinity and kinetic constants can be followed.
Furthermore, SPR biosensor technology has already been applied for analysing the adsorption of small molecules to artificial membranes immobilised on sensor chips and for measuring the influence of compounds on metabolic pathways.
Our service:
- qualitatively ranking of drugs according their binding to plasma proteins (Hit selection)
- detailed compound characterization of high binding drugs (KD, rate constants, %bound value)
For further information, please get in contact with one of our SPR application specialists - we will be pleased to provide you with an individual offer.
Tel.: +49 (0) 561-804 4661 | Fax: +49 (0) 561-804 4665 | info@biaffin.de
We value your privacy
In order to optimize our website for you and to be able to continuously improve it, we use cookies. Further information is available in our Data Protection Statement. Here you can find our Legal Information.