Thermodynamic Interaction analysis
Are you interesting in a complete understanding of the dynamic processes underlying the biomolecular interaction ?
We provide thermodynamic insights into the binding mechanism of your biomolecules.
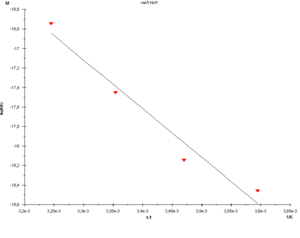
Measurements of binding thermodynamics extends the characterisation of biomolecular intercations, since binding affinity is a function of two quantities: the enthalpy (ΔH) and entropy (ΔS). By exploiting binding thermodynamics the potential of enthalpy and entropy correlations associated with chemical modifications in different regions of the lead molecule can be specified enabling a more robust and reliable drug discovery process.
Biaffin uses a Biacore T100 / T200 instrument to analyze binding kinetics of a defined interaction between two biomolecules at a series of temperatures between 4°C and 40°C. Evaluation of interaction data obtained at varying temperatures provides van't Hoff and Eyring plots which yield thermodynamic constants for the equilibrium and transition state formation.
Please contact our application specialists for getting detailed information and an individual quote.
Tel.: +49 (0) 561-804 4661 | Fax: +49 (0) 561-804 4665 | info@biaffin.de
We value your privacy
In order to optimize our website for you and to be able to continuously improve it, we use cookies. Further information is available in our Data Protection Statement. Here you can find our Legal Information.